Induced Dipoles Incorporated into All-Atom Zn Protein Simulations with Multiscale Modeling
Abstract
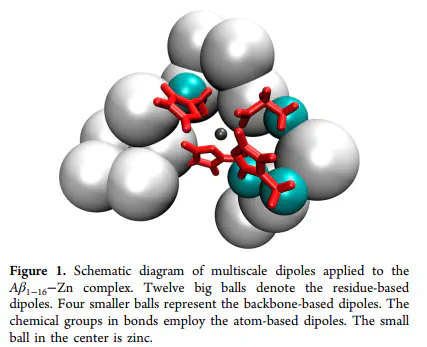
Zinc is found saturated in the deposited Amyloid-beta (Aβ) peptide plaques in Alzheimer’s disease (AD) patients’ brains. Binding of zinc promotes aggregation of Aβ, including the pathogenic aggregates. Up to now, only the region 1-16 of Aβ complexed with zinc (Aβ(1-16)-Zn) is defined structurally in experiment. In order to explore the induced polarization effect of zinc on the global fluctuations and the experimentally observed coordination mode of Aβ(1-16)-Zn, we consider an all-atom molecular dynamics (MD) of Aβ(1-16)-Zn solvated in implicit water. In our model, the zinc polarization affects the whole peptide. The induced dipoles are divided into three distinct scales according to their distances from zinc. Besides, the atomistic polarizability on the coordinating side chains is rescaled to describe the electron redistribution effect. We show that, associated with proper van der Waals (vdW) parameters, our model not only obtains the reasonable coordinating configuration of zinc binding site but also retains the global stabilization, especially the N-terminal region, of the Aβ(1-16)-Zn. We suggest that it is the induced polarization effect that promotes reasonable solvent exposures of hydrophobic/hydrophilic residues regarding zinc-induced Aβ aggregation.