Optimal microdomain crosstalk between endoplasmic reticulum and mitochondria for Ca21 oscillation
Abstract
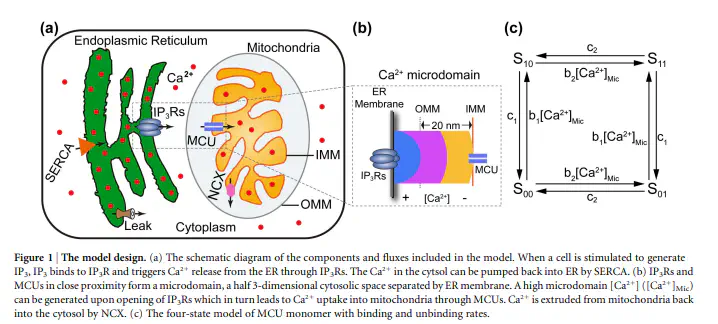
A Ca(2+) signaling model is proposed to consider the crosstalk of Ca(2+) ions between endoplasmic reticulum (ER) and mitochondria within microdomains around inositol 1, 4, 5-trisphosphate receptors (IP3R) and the mitochondrial Ca(2+) uniporter (MCU). Our model predicts that there is a critical IP3R-MCU distance at which 50% of the ER-released Ca(2+) is taken up by mitochondria and that mitochondria modulate Ca(2+) signals differently when outside of this critical distance. This study highlights the importance of the IP3R-MCU distance on Ca(2+) signaling dynamics. The model predicts that when MCU are too closely associated with IP3Rs, the enhanced mitochondrial Ca(2+) uptake will produce an increase of cytosolic Ca(2+) spike amplitude. Notably, the model demonstrates the existence of an optimal IP3R-MCU distance (30-85 nm) for effective Ca(2+) transfer and the successful generation of Ca(2+) signals in healthy cells. We suggest that the space between the inner and outer mitochondria membranes provides a defense mechanism against occurrences of high [Ca(2+)]Cyt. Our results also hint at a possible pathological mechanism in which abnormally high [Ca(2+)]Cyt arises when the IP3R-MCU distance is in excess of the optimal range.