Synonymous mutations in oncogenesis and apoptosis versus survival unveiled by network modeling
Abstract
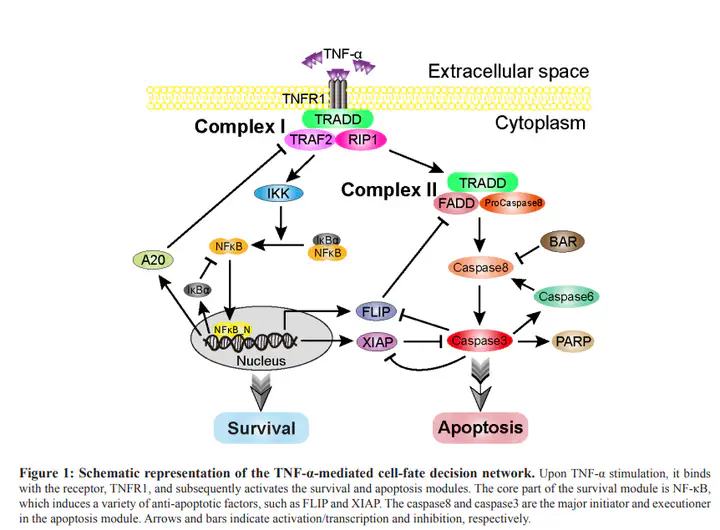
Synonymous mutations, which do not alter the encoded amino acid, have been routinely assumed to be ’neutral’ and would have no effect on phenotype or fitness. Yet increasing observations have emerged to overturn this conventional concept. However, convicted elucidation of how synonymous mutations exert biological consequences in oncogenesis is still lacking. By performing systematic analysis of the TNF-α signaling network model, we identify the critical dose which separates the cell survival and apoptosis regions and define the sensitive parameters with single-parameter sensitivity analysis. Combining with the cancer-related mutation spectra obtained from 9 cancers, our results hint that, similar as missense and nonsense mutations, synonymous mutations are also strongly correlated with the parameter sensitivity of the critical dose, providing possible causal mechanism of the mutations in cancer development. Based on such a correlation, we furthermore dissect that members of caspases family proteases (caspase3, 6, 8) could jointly inhibit NFκB activation, providing efficient pro-apoptotic behavior. Thus, we argue that apoptosis module could suppress survival module through negative feedback of caspases family on NFκB.