Data-Driven Modeling Identifies TIRAP-Independent MyD88 Activation Complex and Myddosome Assembly Strategy in LPS/TLR4 Signaling
Abstract
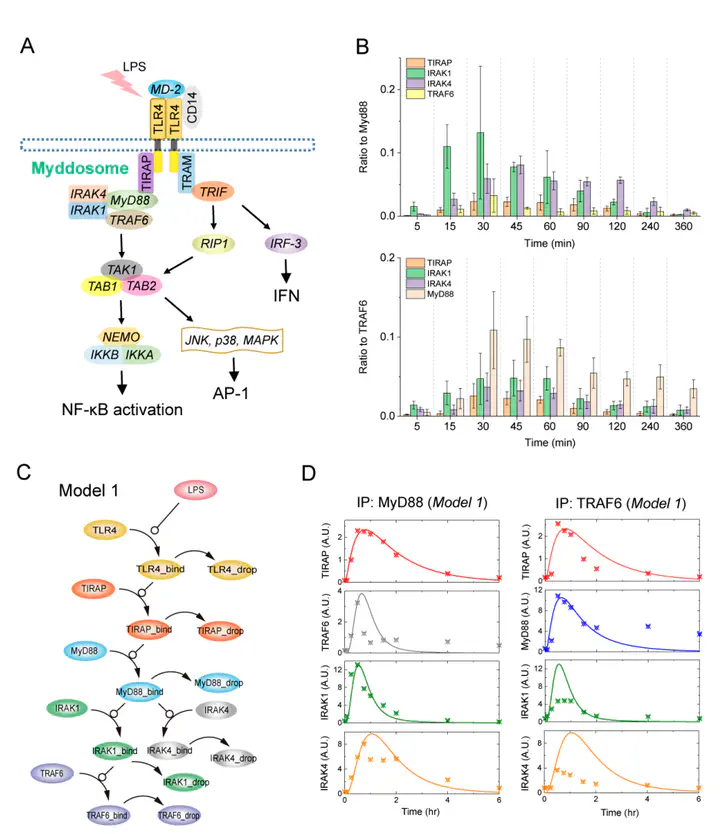
TLR4 complexes are essential for the initiation of the LPS-induced innate immune response.The Myddosome, which mainly contains TLR4, TIRAP, MyD88, IRAK1/4 and TRAF6 proteins,is regarded as a major complex of TLR4.Although the Myddosome has been well studied, a quantitative description of the Myddosome assembly dynamics is still lacking Furthermore, whether some unknown TLR4 complexes exist remains unclear. In this study, we constructed a SWATH-MS data-based mathematical model that describes the component assembly dynamics of TLR4 complexes.In addition to Myddosome, we suggest that a TIRAP-independent MyD88 activation complex is formed upon LPS stimulation, in which TRAF6 is not included. Furthermore, quantitative analysis reveals that the distribution of components in TIRAP-dependent and -independent MyD88 activation complexes are LPS stimulation-dependent. The two complexes compete for recruiting IRAK1/4 proteins. MyD88 forms higher-order assembly in the Myddosome and we show that the strategy to form higher-order assembly is also LPS stimulation-dependent. MyD88 forms a long chain upon weak stimulation, but forms a short chain upon strong stimulation. Higher-order assembly of MyD88 is directly determined by the level of TIRAP in the Myddosome, providing a formation mechanism for efficient signaling transduction. Taken together, our study provides an enhanced understanding of component assembly dynamics and strategies in TLR4 complexes