Cell death modes are specified by the crosstalk dynamics within pyroptotic and apoptotic signaling
Abstract
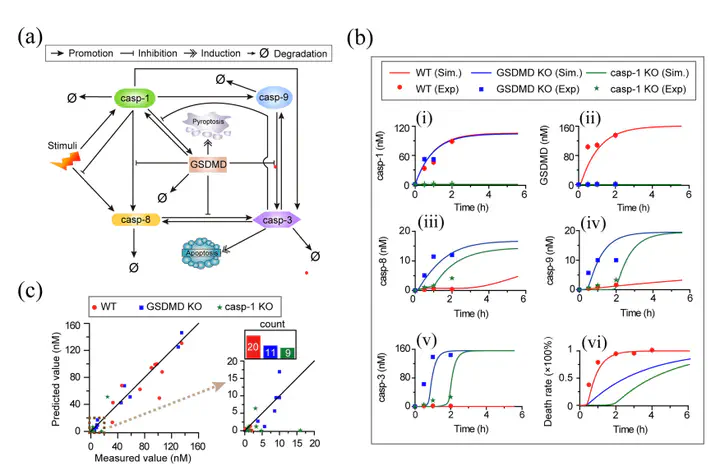
The crosstalk between pyroptosis and apoptosis pathways plays crucial roles in homeostasis, cancer, and other pathologies. However, its molecular regulatory mechanisms for cell death decision-making remain to be elucidated. Based on the recent experimental studies, we developed a core regulatory network model of the crosstalk between pyroptosis and apoptosis pathways. Sensitivity analysis and bifurcation analysis were performed to assess the death mode switching of the network. Both the approaches determined that only the level of caspase-1 or gasdermin D (GSDMD) has the potential to individually change death modes. The decrease of caspase-1 or GSDMD switches cell death from pyroptosis to apoptosis. Seven biochemical reactions among the 21 reactions in total that are essential for determining cell death modes are identified by using sensitivity analysis. While with bifurcation analysis of state transitions, nine reactions are suggested to be able to efficiently switch death modes. Monostability, bistability, and tristability are observed under different conditions. We found that only the reaction that caspase-1 activation induced by stimuli can trigger tristability. Six and two of the nine reactions are identified to be able to induce bistability and monostability, respectively. Moreover, the concurrence of pyroptosis and apoptosis is observed not only within proper bistable ranges,but also within tristable ranges, implying two potentially distinct regulatory mechanisms. Taken together, this work sheds new light on the crosstalk between pyroptosis and apoptosis and uncovers the regulatory mechanisms of various stable state transitions, which play important roles for the development of potential control strategies for disease prevention and treatment.