Network modeling-based identification of the switching targets between pyroptosis and secondary pyroptosis
Abstract
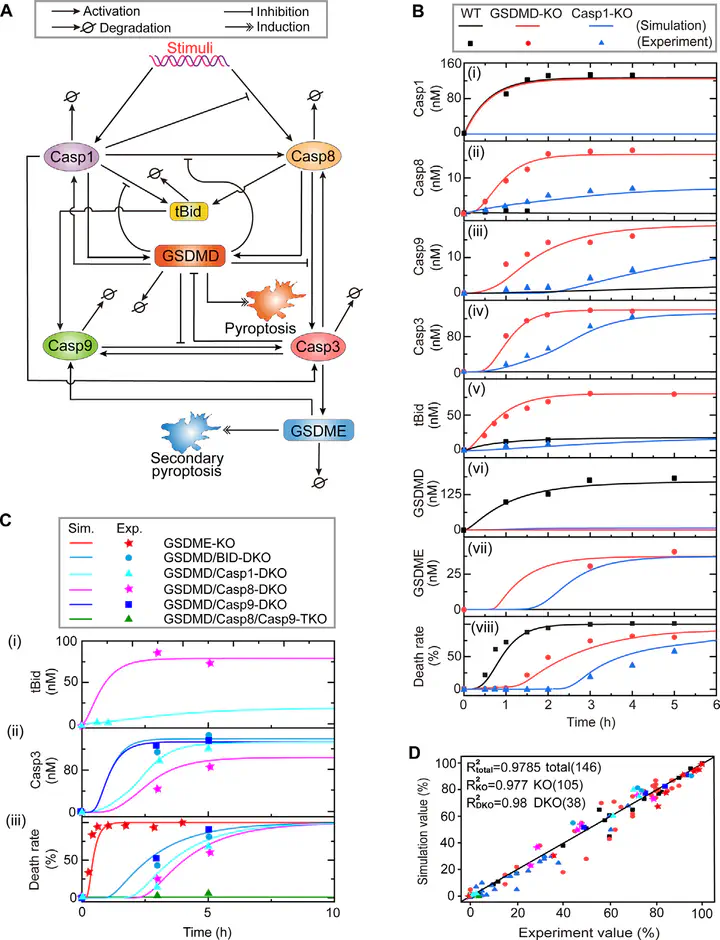
The newly identified cell death type, pyroptosis plays crucial roles in various diseases. Most recently, mounting evidence accumulates that pyroptotic signaling is highly correlated with coronavirus disease 2019 (COVID-19). Thus, understanding the induction of the pyroptotic signaling and dissecting the detail molecular control mechanisms are urgently needed. Based on recent experimental studies, a core reg ulatory model of the pyroptotic signaling is constructed to investigate the intricate crosstalk dynamics between the two cell death types, i.e., pyroptosis and secondary pyroptosis. The model well reproduces the experimental observations under different conditions. Sensitivity analysis determines that only the expression level of caspase-1 or GSDMD has the potential to individually change death modes. The de crease of caspase-1 or GSDMD level switches cell death from pyroptosis to secondary pyroptosis. Be sides, eight biochemical reactions are identified that can efficiently switch death modes. While from the viewpoint of bifurcation analysis, the expression level of caspase-3 is further identified and twelve bio chemical reactions are obtained. The coexistence of pyroptosis and secondary pyroptosis is predicted to be observed not only within the bistable range, but also within proper monostable range, presenting two potential different control mechanisms. Combined with the landscape theory, we further explore the stochastic dynamic and global stability of the pyroptotic system, accurately quantifying how each compo nent mediates the individual occurrence probability of pyroptosis and secondary pyroptosis. Overall, this study sheds new light on the intricate crosstalk of the pyroptotic signaling and uncovers the regulatory mechanisms of various stable state transitions, providing potential clues to guide the development for prevention and treatment of pyroptosis-related diseases