Specificity and competition of mRNAs dominate droplet pattern in protein phase separation
Abstract
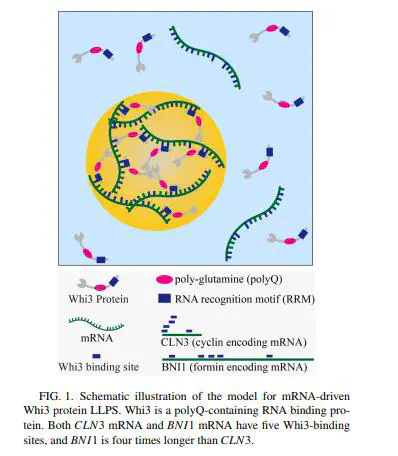
Phase separation is a ubiquitous and emerging mechanism underlying intracellular organization. Yet how distinct molecular compositions in phase-separated condensates are established and what prohibits these condensates from randomly fusing together remain largely unanswered. Here, through proposing a Cahn-Hilliard phase-field model paired with Ginzburg-Landau free-energy scheme, we explored how compositions are assembled in messenger RNAs (mRNAs)-driven protein droplets with respect to distinct physical properties. By analyzing the intradroplet heteropatterning of two specific droplets mRNA-driven Whi3 droplets), we demonstrated that the growth rate of droplet size and higher-order complexes assembly in droplet are severally determined by the diffusion rate of droplet and the binding rate of mRNA with protein. While considering the three-species mixed system of two mRNAs that share a common binding protein, the two specific droplets preferentially assemble separately rather than colocalize. We found the two droplets compete with each other by snatching the protein that has been recruited into the other droplet when the free protein is insufficient. Further analysis dissects that the gradient–interfacial energy coefficients, initial mRNAs levels, and mRNAs-protein binding rates can efficiently shift the spatial patterns of the two specific droplets from segregation to the shared interface or enclosed patterns. All three patterns obtained by tuning the initial mRNAs levels or mRNA-protein binding rates have been experimentally observed. Another unreported core-shell pattern is predicted by synergistically reducing the gradient and interfacial energy coefficients. Our findings shed light on the establishment of intradroplet compositions in condensates with distinct physical properties and the general control mechanisms of phase-separated pattern formation.